Iso 11607 top
Iso 11607 top, ISO 11607 1 and Other Requirements top
$96.00
SAVE 50% OFF
$48.00
$0 today, followed by 3 monthly payments of $16.00, interest free. Read More
Iso 11607 top
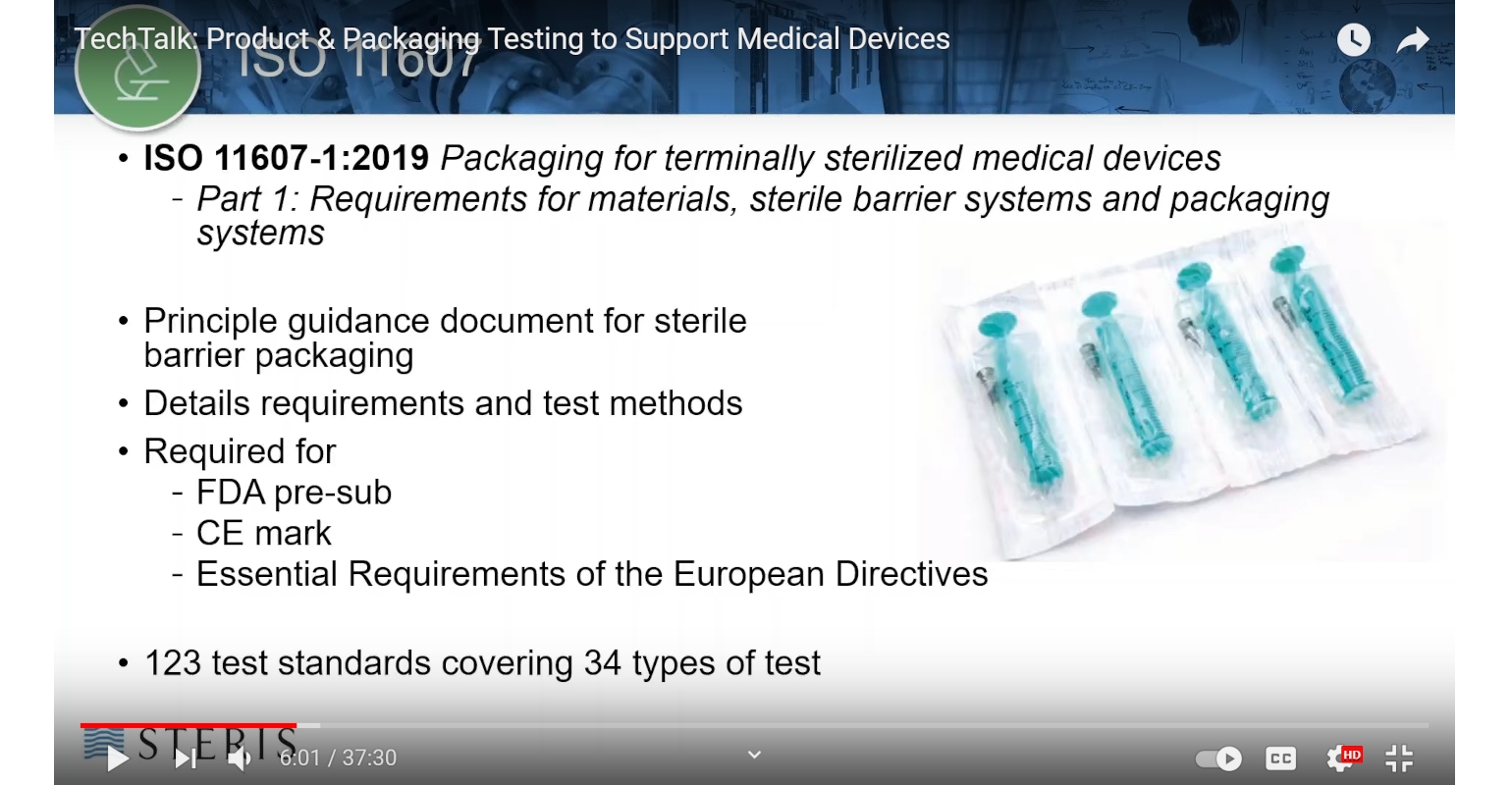
ISO 11607 1 and Other Requirements
Packaging Validation of Medical Devices Impact of the Revisions of ISO 11607 Suitable Strategies
Medical Device Packaging Insider Tips Must See Videos
ISO 11607 1 2006 Packaging for terminally sterilized medical
ISO 11607 Part IIshort MSU MediaSpace
ISO 11607 1 Medical Device Package Testing Keystone Compliance
Description
Product Name: Iso 11607 top
ISO 11607 1 2019 Amd1 2023 Amendment 1 Packaging for top, Learning Share Clip ISO 11607 Facebook top, EN ISO 11607 1 2020 Packaging for terminally sterilized medical top, 23 Other standards referenced by ISO 11607 1 2 Download top, AAMI ISO 11607 1 2019 top, ISO 11607 1 2019 Emballages des dispositifs m dicaux st rilis s au stade terminal top, ISO 11607 2 Packaging for Final Sterilized Medical Devices Part top, BS EN ISO 11607 1 2009 Packaging for terminally sterilized top, ISO 11607 1 Amend 2014 07 PDF International Organization top, Packaging 101 ISO 11607 top, ISO 11607 A Primer on Packaging for Terminally Sterilized Medical top, ISO 11607 1 Packaging for Final Sterilized Medical Devices Part top, ISO 11607 1 Packaging Testing for Sterilized Medical Devices top, Certificate MuDanJiang Plasma Physical Application Technology Co top, ISO 11607 1 and Other Requirements top, Packaging Validation of Medical Devices Impact of the Revisions of ISO 11607 Suitable Strategies top, Medical Device Packaging Insider Tips Must See Videos top, ISO 11607 1 2006 Packaging for terminally sterilized medical top, ISO 11607 Part IIshort MSU MediaSpace top, ISO 11607 1 Medical Device Package Testing Keystone Compliance top, Anecto iso 11607 2011 PPT top, ABNT NBR ISO 11607 1 NBRISO11607 1 Embalagem final para produtos top, ISO 11607 1 2019 top, Life Science Outsourcing Inc. on LinkedIn medicalexcellence top, ISO 11607 2 2019 Amd 1 2023 En PDF Risk Management Risk top, Medical Device Packaging Insider Tips Must See Videos top, ISO 11607 1 2018 top, UNE CEN ISO TS 16775 2021 Packaging for terminally sterilized medical devices Guidance on the application of ISO 11607 1 and ISO 11607 2 ISO TS top, ISO 11607 1 2019 Packaging for terminally sterilized medical top, nueva norma EN ISO 11607 1 2017 y 2 gu a sellado top, Anecto iso 11607 2011 PPT top, Packaging Validation according to ISO PDF Free Download top, ISO 11607 1 2019 Amd 1 2023 top, Design Terminally Sterile Packaging 3 Ways to Prevent ISO 11607 top, ISO 11607 2 2019 top.
ISO 11607 1 2019 Amd1 2023 Amendment 1 Packaging for top, Learning Share Clip ISO 11607 Facebook top, EN ISO 11607 1 2020 Packaging for terminally sterilized medical top, 23 Other standards referenced by ISO 11607 1 2 Download top, AAMI ISO 11607 1 2019 top, ISO 11607 1 2019 Emballages des dispositifs m dicaux st rilis s au stade terminal top, ISO 11607 2 Packaging for Final Sterilized Medical Devices Part top, BS EN ISO 11607 1 2009 Packaging for terminally sterilized top, ISO 11607 1 Amend 2014 07 PDF International Organization top, Packaging 101 ISO 11607 top, ISO 11607 A Primer on Packaging for Terminally Sterilized Medical top, ISO 11607 1 Packaging for Final Sterilized Medical Devices Part top, ISO 11607 1 Packaging Testing for Sterilized Medical Devices top, Certificate MuDanJiang Plasma Physical Application Technology Co top, ISO 11607 1 and Other Requirements top, Packaging Validation of Medical Devices Impact of the Revisions of ISO 11607 Suitable Strategies top, Medical Device Packaging Insider Tips Must See Videos top, ISO 11607 1 2006 Packaging for terminally sterilized medical top, ISO 11607 Part IIshort MSU MediaSpace top, ISO 11607 1 Medical Device Package Testing Keystone Compliance top, Anecto iso 11607 2011 PPT top, ABNT NBR ISO 11607 1 NBRISO11607 1 Embalagem final para produtos top, ISO 11607 1 2019 top, Life Science Outsourcing Inc. on LinkedIn medicalexcellence top, ISO 11607 2 2019 Amd 1 2023 En PDF Risk Management Risk top, Medical Device Packaging Insider Tips Must See Videos top, ISO 11607 1 2018 top, UNE CEN ISO TS 16775 2021 Packaging for terminally sterilized medical devices Guidance on the application of ISO 11607 1 and ISO 11607 2 ISO TS top, ISO 11607 1 2019 Packaging for terminally sterilized medical top, nueva norma EN ISO 11607 1 2017 y 2 gu a sellado top, Anecto iso 11607 2011 PPT top, Packaging Validation according to ISO PDF Free Download top, ISO 11607 1 2019 Amd 1 2023 top, Design Terminally Sterile Packaging 3 Ways to Prevent ISO 11607 top, ISO 11607 2 2019 top.